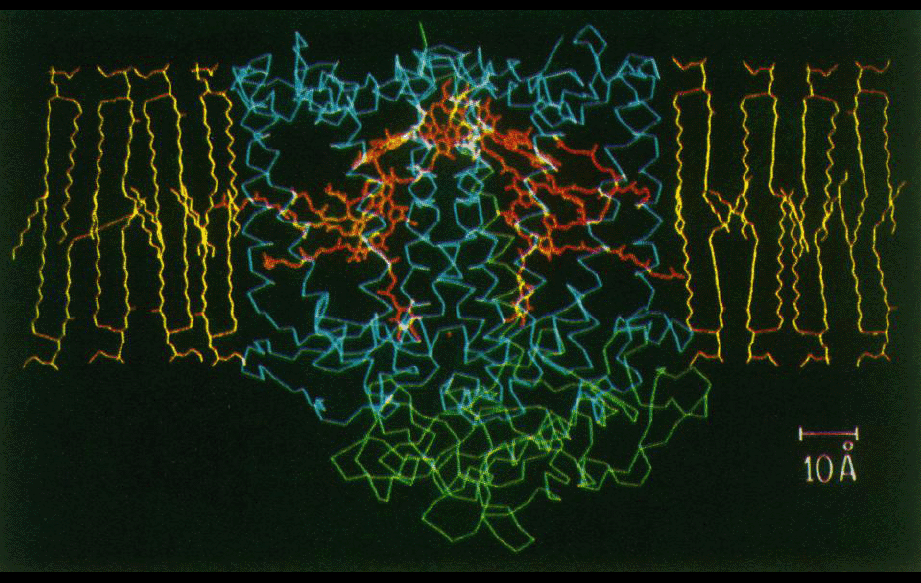
Beginning around 1984, while working as a postdoc with George Feher at UCSD, Jim Allen was able to grow good single crystals of the bacterial photosynthetic reaction center (RC) from Rb. sphaeroides. A collaboration was initiated with Doug Rees’ lab at UCLA, where we participated in the crystallographic work. No structures had been determined yet of transmembrane proteins, but in Germany, Deisenhofer, Michel, and Huber were already further along with their work on the homologous complex from Rps. viridis. Their tracing of the RC structure was reported in 1984, and refined in 1985. Structures of the sphaeroides RC based on Jim Allen’s crystals were reported beginning in 1987. Although the sphaeroides RC was the second transmembrane protein structure reported, it provided some of the first analyses of protein-lipid interactions and structural properties of a protein in its membrane environment, and of electrostatics of large proteins in a non-uniform dielectric medium.
Programs written by Doug were used to analyze what regions of the transmembrane protein complex were likely to be in contact with the lipid bilayer (figures middle and bottom right). The amino acid composition of the transmembrane surface of the reaction center was analyzed. It was also noted that the amino acid sequence variability between homologs was higher in the lipid-facing residues compared to the residues in the protein interior.
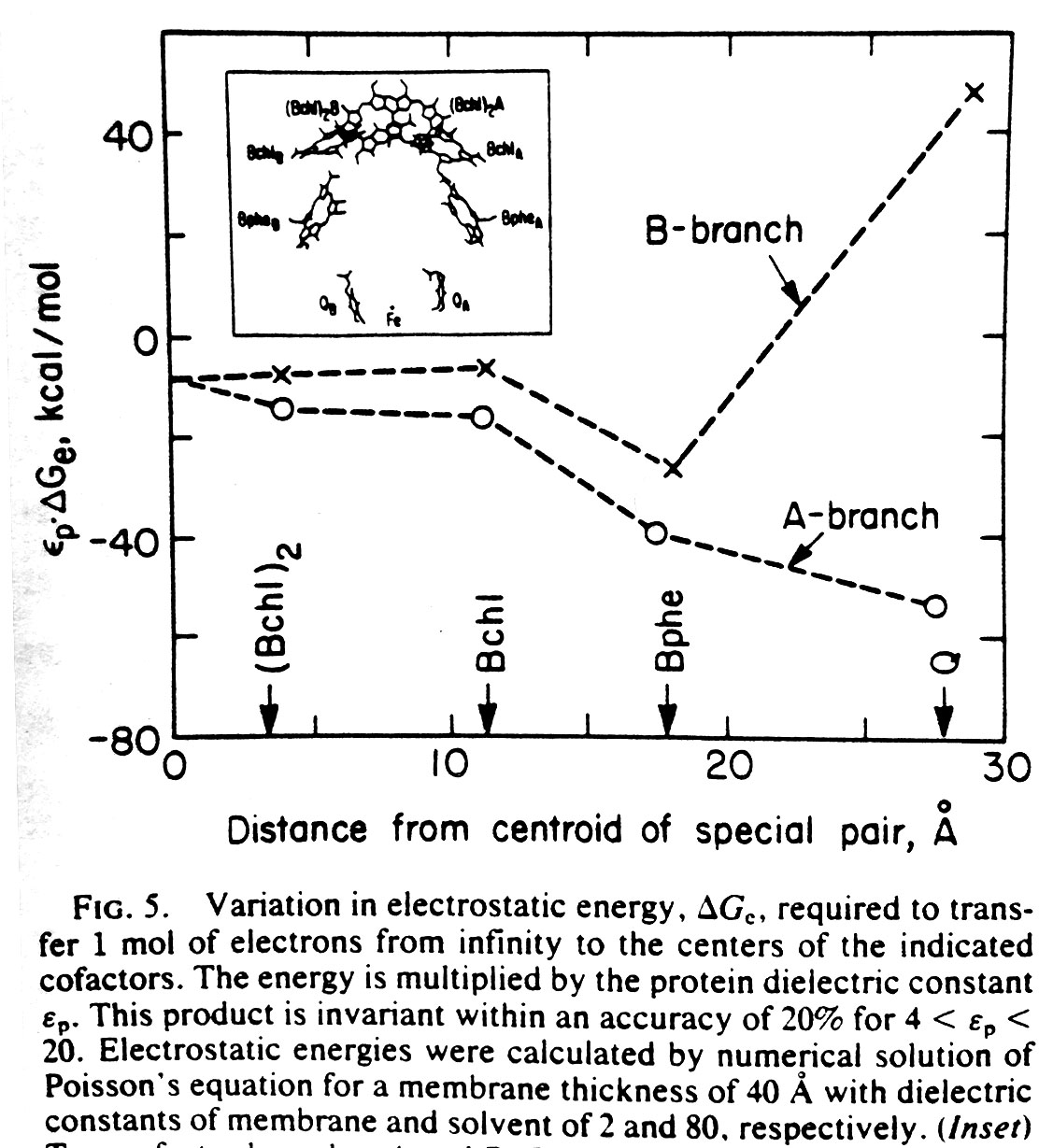
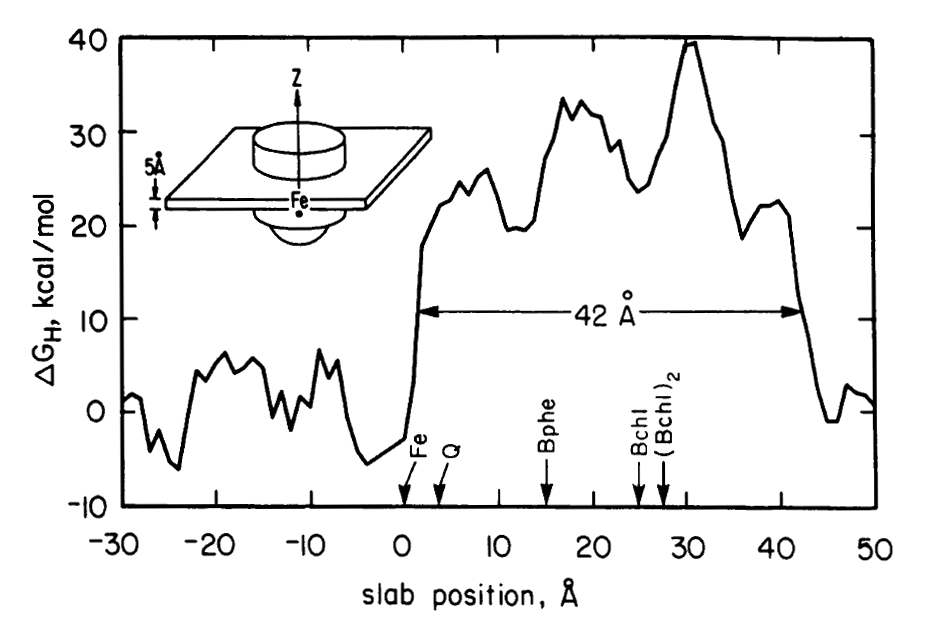
Electrostatics in the transmembrane reaction center were also a key point of interest. The electron ejected by the ‘special pair’ of chlorophyl molecules traveled down only one of the two nearly equivalent cofactor branches, which suggested the possible role of electrostatic differences. It was recognized by this point (and emphasized by Gilson and Honig and others) that simple application of Coulomb’s law for protein electrostatics would not be valid since the dielectric for the protein interior was very much lower than the solvent — and the case of the reaction center was further complicated by the even lower dielectric of the membrane environment. Finite difference equations had been developed by Warwicker and Watson for solving Poisson’s equation on a grid in a way that would allow non-uniform dielectric values to be accommodated. But the computational time for solving such equations depends strongly on the size of the system, making the calculation nearly intractable for large protein systems. In 1986, Klapper et al. (Proteins 1, 47-59) used a multi-gridding approach in which the calculations were performed first on a coarse grid and then progressively improved with calculations on finer grids. For our 1987 paper on electrostatics in the reaction center (PNAS 84, 6438-42). we instead used a powerful numerical approach called successive overrelaxation to achieve rapid convergence of the Poisson-Boltzmann equation on a fine grid, following equations that appeared a year earlier in the instant classic, Numerical Recipes. Limited details of our 1987 calculation were provided in the PNAS paper owing to strict page limits. More detailed discussion of using successive overrelaxation to solve the Poisson-Boltzmann equation was given in 1991 by Nicholls and Honig (J. Comp. Chem. 12, 435-445).
In the Rees lab, subsequent work on the reaction center was carried out by Hiromi Komiya, Art Chirino, Michael Stowell, Herb Axelrod, Barbara Hsu, and others. Diesenhofer, Michel, and Huber earned the Nobel Prize in Chemistry in 1998 for their work on the reaction center structure.